The goal in the Life Sciences industry is to cure all diseases – while at the same time, reducing the cost of the medication that we all need to stay healthy. How is this accomplished with today’s challenges of doing more with less?
A recent article from PwC titled 2017 Pharmaceuticals and Life Sciences Industry Trends shines some light on what’s currently in motion:
“Pharmaceuticals and life sciences companies are experiencing a wave of competing challenges…” It continues stating, “some pharma companies are positioned to capitalize on this situation — to demonstrate the clinical and economic value of their products and solutions in fresh ways. These companies have begun to adopt new technologies for data analytics that can manage and assess the results of personalized medicine delivery and determine the direction of product development.”
https://www.strategyand.pwc.com/media/file/2017-Pharmaceuticals-and-Life-Sciences-Industry-Trends.pdf
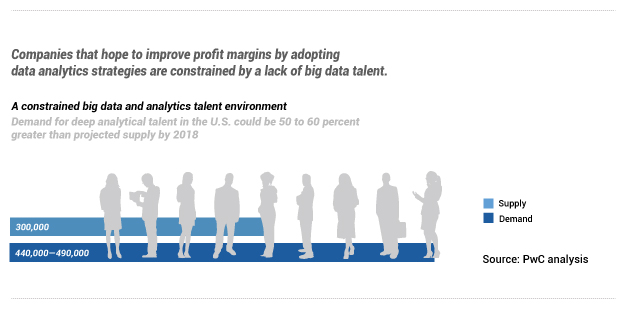
The report then highlights that the, “demand for deep analytical talent in the U.S. could be 50 to 60 percent greater than projected supply by 2018”. If the business side of these life science organizations are demanding data talent, then one could expect to see demand for the supporting infrastructure.
Outside of the 21 CRF Part 11 compliance2 (and other regulations), that means data integrity has become even more significant across the supply chain because the future of the business and product are being developed based on this newly available Big Data.
This especially extends out to the manufacturing and processing side of the equation. Data sources from industrial automation like batch control systems, materials handling, packaging, MES, HMI, Historian, lab systems and many other sources all come into play. All are potential insights into how that product was developed and could be developed in the future. Another is typically how data on the manufacturing side is leveraged today – for operational efficiency gains and higher yields from process optimization.
Integrity of Data and Systems
It’s clear that data and system integrity is increasingly growing it terms of importance outside of just compliance. So, how does a team protect themselves from data and system integrity challenges?
Avoid These:
- Mixed levels and partial automation – Updating an entire infrastructure at one time can be overwhelming, but making smaller upgrades with the bigger picture in mind can set the stage for a cohesive system.
- Disconnected automation and control systems – Supporting consistent communication among systems is imperative in preventing data loss.
- Lack of central recipe libraries – Consistency is key – especially with data integrity. Make sure systems have a strong base rule book that guides all processes.
- Manual and paper processes and batch records – Manual and paper processes are risky to rely on while digitalized data can live forever.
- Hardware with less than eight-year lifecycles – Refreshes are inconvenient, time consuming and expensive. Invest in systems that can last.
Consider consolidating these systems onto one platform where your team can virtualize all your applications and systems, allowing for one central source of data to ensure maximum data and system integrity.
Learn more about supporting your Pharma and Life Sciences data and processes with this on-demand webinar.